
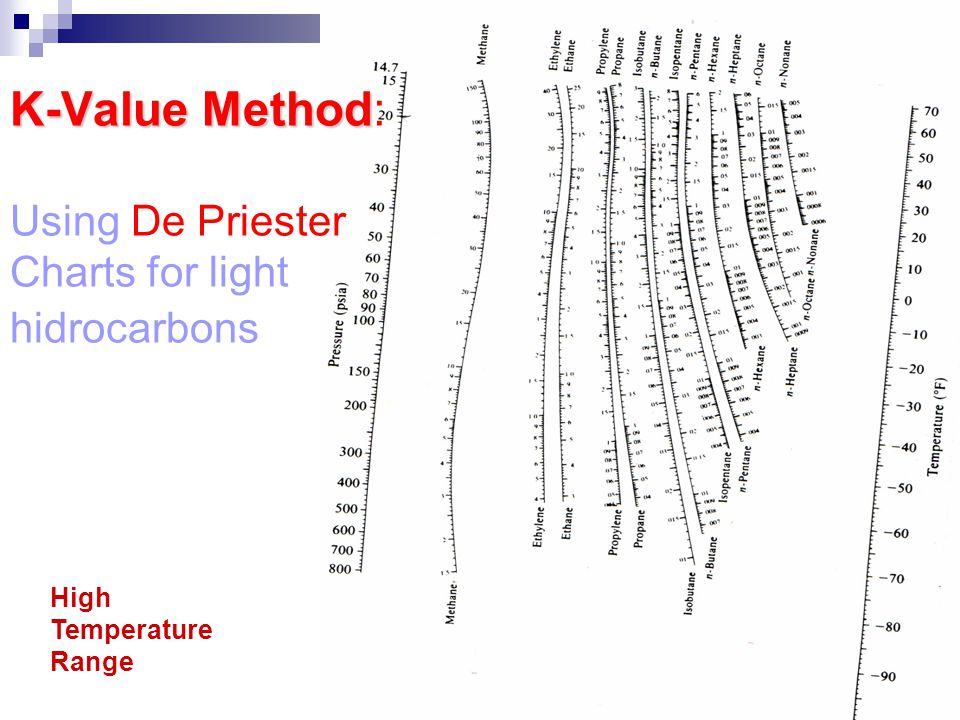
SI versions of these charts have been developed by Dadyburjor. For K as a function of T and P only, the DePriester charts provide good starting values for the iteration.
One cannot calculate K values until phase compositions are known, and those cannot be known until the K values are available to calculate them. Ī trial-and- error procedure is required with any K-value correlation that takes into account the effect of composition.

The Kellogg charts, and hence the DePriester charts, are based primarily on the Benedict-Webb-Rubin equation of state, which can represent both the liquid and the vapor phases and can predict K values quite accurately when the equation constants are available for the components in question. These charts are a simplification of the Kellogg charts and include additional experimental data. The easiest to use are the DePriester charts, which cover 12 hydrocarbons (methane, ethylene, ethane, propylene, propane, isobutane, isobutylene, /i-butane, isopentane, /1-pentane, /i-hexane, and /i-heptane). For example, several major graphical i light-hydrocarbon systems. However, for mixtures of compounds of similar molecular structure and size, the K value depends mainly on temperature and pressure. 4, the i complex function of temperature, pressure, and equilibrium vapor- and hquid-phase compositions. Other Equilibrium Diagrams: P = 1.As discussed in Sec.

Phase Equilibrium (Alternate Form VLE) Historically, when estimates were done by hand: Sometimes the K values are nearly composition independent “hand” techniques of design/solution have used DePriester Charts (hydrocarbons):ĭePriester Chart P = 2 bar T = 100 oC Isobutane others…. Phase Equilibrium (Alternate Form VLE) Historically, when estimates were done by hand:


 0 kommentar(er)
0 kommentar(er)
